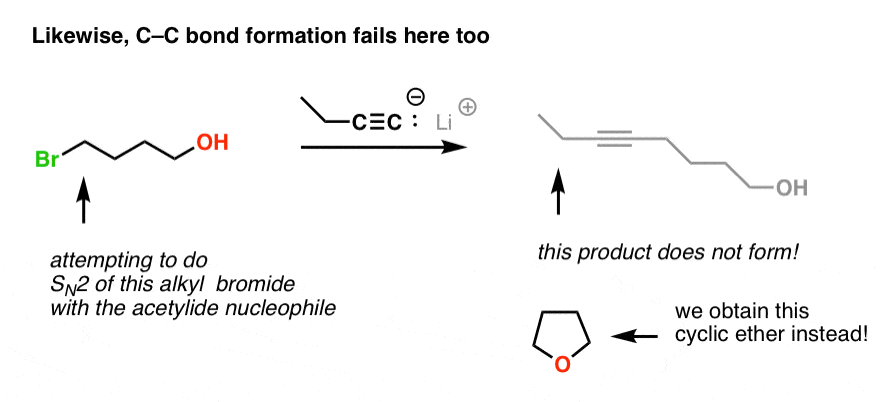
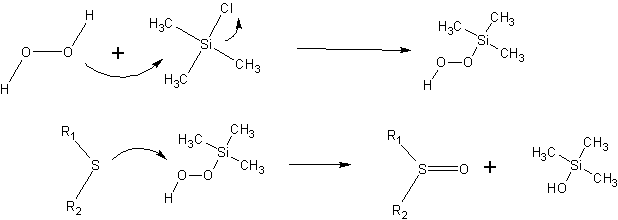
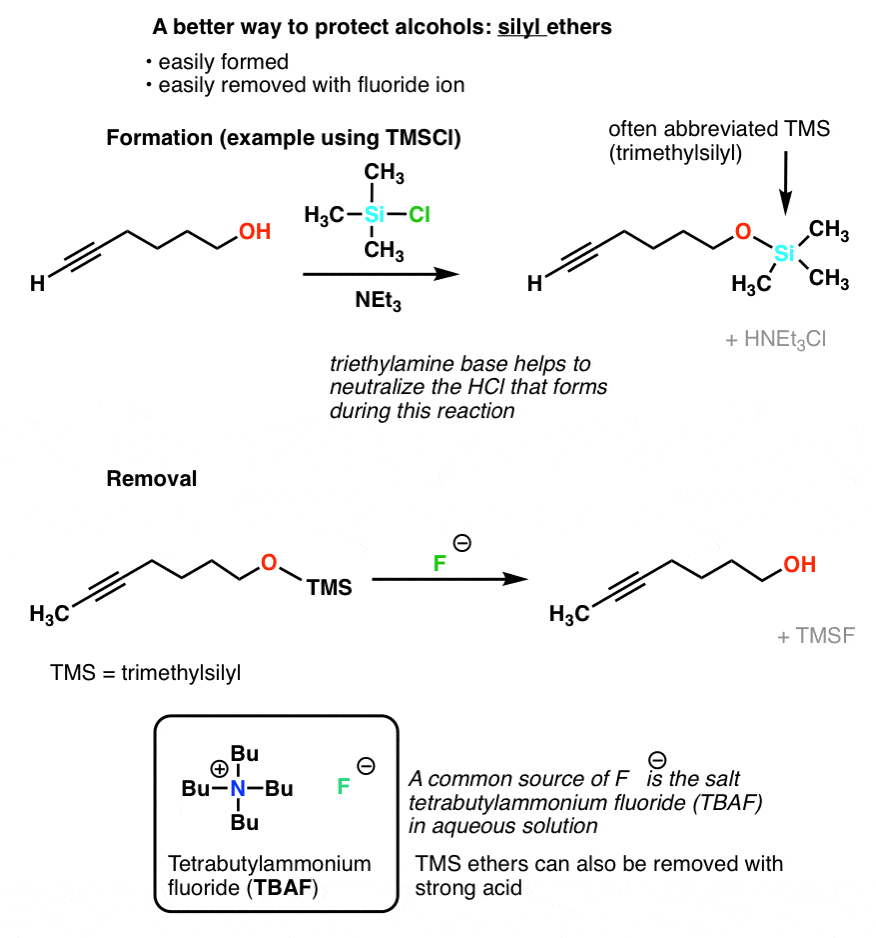
PDF) TMSCl mediated highly efficient one-pot synthesis of octahydroquinazolinone and 1,8-dioxo-octahydroxanthene derivatives

Scheme 1. (a) MMTrCl/Py; (b) TMSCl/Py; (c) BzCl or Ac 2 O or (ClCH 2... | Download Scientific Diagram

Revisiting the Mg/TMSCl/Dipolar Solvent System for Dearomatic Silylation of Aryl Carbonyl Compounds: Substrate Scope, Transformations, and Mechanistic Studies | The Journal of Organic Chemistry
TMSCl as a Mild and Effective Source of Acidic Catalysis in Fischer Glycosidation and Use of Propargyl Glycoside for Anomeric Pr

TRIMETHYLSILYL CHLORIDE CATALYZED SYNTHESIS OF SUBSTITUTED BENZIMIDAZOLES USING TWO PHASE SYSTEM UNDER MICROWAVE CONDITIONS, AND THEIR ANTIMICROBIAL STUDIES

Revisiting the Mg/TMSCl/Dipolar Solvent System for Dearomatic Silylation of Aryl Carbonyl Compounds: Substrate Scope, Transformations, and Mechanistic Studies | The Journal of Organic Chemistry

Mild Deprotection of Dithioacetals by TMSCl/NaI Association in CH3CN - Yao - 2020 - European Journal of Organic Chemistry - Wiley Online Library

PDF) TMSCl mediated highly efficient one-pot synthesis of octahydroquinazolinone and 1,8-dioxo-octahydroxanthene derivatives

Metal-Free Synthesis of 4-Chloroisocoumarins by TMSCl-Catalyzed NCS-Induced Chlorinative Annulation of 2-Alkynylaryloate Esters | The Journal of Organic Chemistry

Mild Deprotection of Dithioacetals by TMSCl/NaI Association in CH3CN - Yao - 2020 - European Journal of Organic Chemistry - Wiley Online Library

TMSCl-catalysed condensation of α-diketone compounds with urea/thiourea derivatives under solvent-free conditions | SpringerLink

Mild Deprotection of Dithioacetals by TMSCl/NaI Association in CH3CN - Yao - 2020 - European Journal of Organic Chemistry - Wiley Online Library

TMSCl-Catalyzed Electrophilic Thiocyano Oxyfunctionalization of Alkenes Using N-Thiocyano-dibenzenesulfonimide | Organic Letters

TMSCl-Catalyzed Electrophilic Thiocyano Oxyfunctionalization of Alkenes Using N-Thiocyano-dibenzenesulfonimide | Organic Letters

Highly Efficient 1,4-Addition of 1,3-Diesters to Conjugated Enones by In/ TMSCl | The Journal of Organic Chemistry

Solvent and Catalyst‐Free Synthesis of Silicon‐Protected Alcohols - Mittersteiner - 2018 - ChemistrySelect - Wiley Online Library